Ceftriaxone Sodium Injection
Ceftriaxone is a third-generation cephalosporin with a wide spectrum of activity. For many different infections, its extended half-life, great tissue penetration, and once-daily administration ease of use make it a recommended choice. It is a recommended treatment for many infections like respiratory tract, urinary system, skin & soft tissue infections, etc. It is available in the market as a white crystalline powder.
Mechanism Of Action:
Ceftriaxone exerts its action by inhibiting bacterial cell wall synthesis. It binds to penicillin-binding proteins (PBPs), which are fundamental for the cross-linking of peptidoglycan layers in the bacterial cell wall. This inhibition causes cell wall weakening, a rise of osmotic pressure, and bacterial cell lysis that leads to cell death.
Ceftriaxone is bactericidal and active against a wide range of Gram-positive and Gram-negative bacteria. Some important ones are Escherichia coli, Streptococcus pneumoniae, Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae. It is more effective against beta-lactamase-producing bacteria.
Uses
Pneumonia, bronchitis (respiratory tract infections)
Meningitis
Urinary tract infections
Uncomplicated gonococcal infections
Sepsis
Surgical prophylaxis
Skin & soft tissue infections
Bone & joint infections
Dosage and Method of Administration
The age, kind of illness, degree, renal/hepatic function of the patient all affect the dosage of ceftriaxone.
Children Above Twelve Years & Adults;
1-2g once daily for mild to moderate infections;
2 g once daily or split in two doses every twelve hours for severe infections.
Meningitis: 2 g IV every 12 hours
Gonorrhea: 250 mg IM once dose
Surgical prophylaxis: 1 -2g IV
Neonates, infants, and children:
Neonates: 20–50 mg/kg per day.
Infants and children (>14 days–12 years): 50–100 mg/kg once a day, maximum 2 g daily.
Dose modification is only needed in severe renal failure; close observation of medication accumulation is essential.
How to reconstitute injection ceftriaxone
Making a Ceftriaxone Solution from Powder
Supplied as a sterile powder, ceftriaxone needs to be reconstituted before use. The method of administration—IV or IM—will determine the diluent choice.
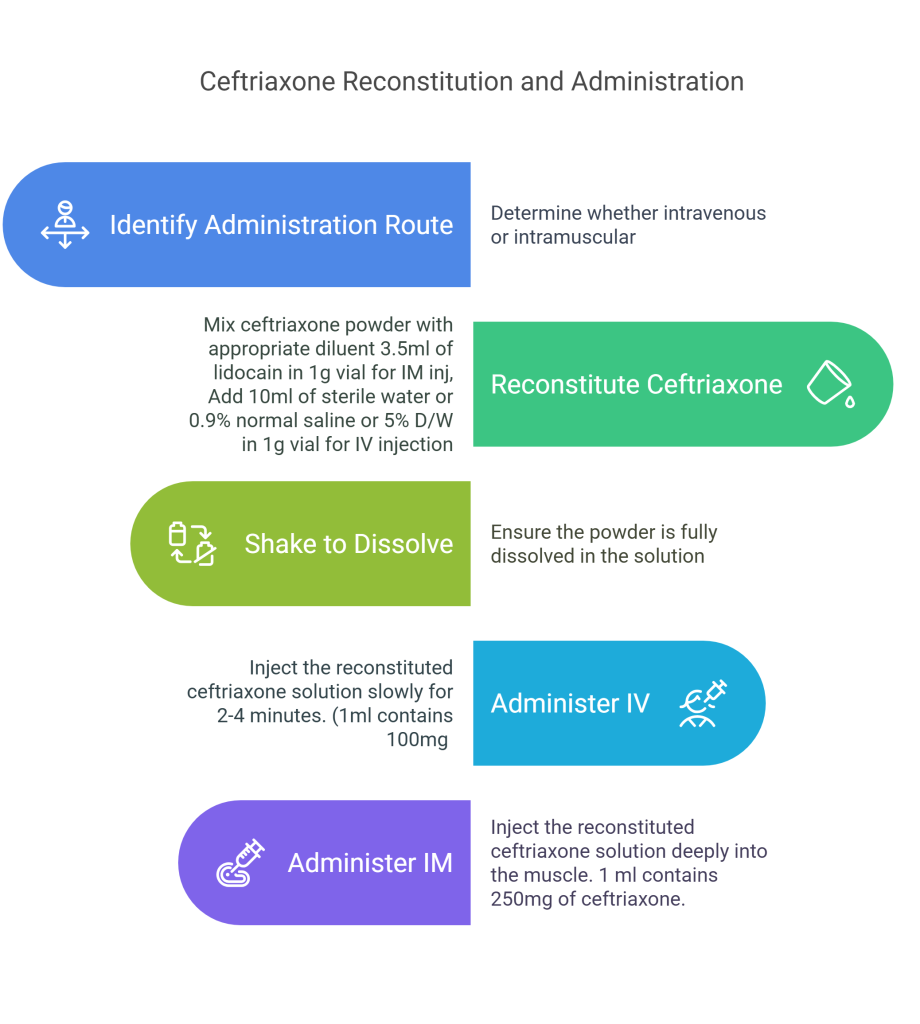
Intravenous Administration
Reconstitute 1 g of ceftriaxone with 10 mL of 0.9% normal saline, 5% dextrose water, or sterile water, or 5 mL to a 500 mg vial.
Shake well to dissolve powder
Inject slowly for about 2-4 minutes.
1 mL of solution contains approximately 100 mg equivalent of ceftriaxone.
Intramuscular Administration
Use 3.5 mL of sterile water for injection or lidocaine 1% (without epinephrine) for a 1 g vial or 2 mL in each 500 mg vial. To minimize injection site pain, use Lidocaine 1%.
Shake well to dissolve powder.
Deeply inject into the muscle.
Never exceed 1 g at one site.
1 mL of solution contains approximately 250 mg equivalent of ceftriaxone.
Adverse Effects;
Typical Side Effects
Gastrointestinal: nausea, vomiting, diarrhea, stomach discomfort
hematologic: eosinophilia, thrombocytosis
dermatologic: rash, pruritus
Pain and induration at IM/IV site
Major Side Effects
Hypersensitivity reactions like anaphylaxis and angioedema
Clostridium difficile-associated diarrhea (CDAD)
Hemolytic anemia
Nephrotoxicity
Stability & Storage
Unreconstituted powder must be stored at room temperature (20–25°C or 68–77°F), covered from light and moisture.
Reconstituted solution
For IV will be stable for 24 hours in a refrigerator
For IM will be stable for 6 hours in a refrigerator
Conclusion
A powerful third-generation cephalosporin with a wide spectrum of activity is Ceftriaxone sodium. For many different infections, its extended half-life, great tissue penetration, and once daily administration easeability make it a recommended choice. To guarantee safe and efficient treatment, however, much attention should be paid to its possible negative effects, compatibility problems, and appropriate reconstitution methods.
